Introduction: The approval of hematological drugs by the US Food and Drug Administration (FDA) relies on rigorous evaluation through pivotal trials. To gain insights into the quality and potential bias in these trials, we conducted a cross-sectional analysis of FDA-approved hematological drugs from 2008 to 2022. This study examines the design characteristics and risk of bias in pivotal trials, aiming to enhance treatment decisions, regulatory processes, and ultimately benefit patients and healthcare professionals.
Methods: We identified all novel hematological drugs approved between 2008 and 2022. We then collected data from publicly available information on the US Food and Drug Administration's Web site and the scientific literature. We assessed approved indications, regulatory characteristics, study design characteristics (randomization, blinding, phase), and risk of bias using the revised Cochrane tool (bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result).
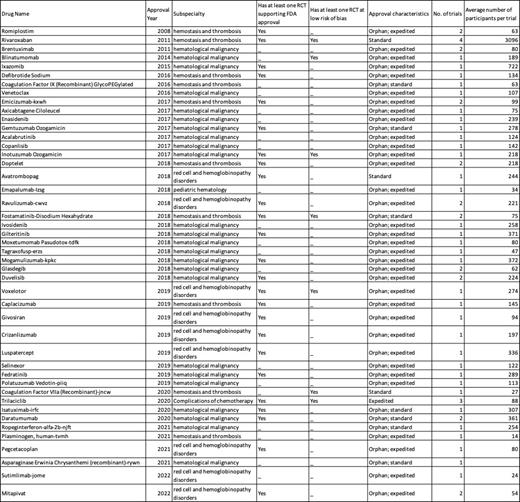
Results: From 2008 to 2022, the FDA approved a total of 45 novel hematological drugs. A total of 24 (53.3%) approvals were indicated for the treatment of hematological malignancies, 10 (22.2%) for hemostasis and thrombosis, 9 (20%) for red cell and hemoglobinopathy disorders, 1 (2.2%) for pediatric hematology, and 1 (2.2%) for other indications. During this period, 41 (91.1%) drugs were approved for orphan indications and 35 (77.8%) drugs were granted expedited approvals.
45 hematological drugs were supported by 60 trials, including 38 phase 3 trials (63.3%). Of these, 34 (56.7%) were randomized controlled trials, 24 (40%) were single-arm studies that evaluated the experimental treatment alone without a comparator, and 2 (3.3%) were non-randomized comparative studies. Of the 34 randomized controlled trials, 17 trials had an active comparator while 17 were placebo-controlled. Only eleven drug approvals were supported by two or more pivotal trials. Adequate masking (blinding) of study group assignment was seen in 22 (64.7%) of the 34 randomized controlled trials; while all the non-randomized pivotal trials were open-label studies.
Fifteen trials were judged to be at high risk of bias owing to the domains of missing outcome data and deviations from intended interventions. 10 of these trials were non-randomized, open-label studies. Moreover, 10 trials were judged to be at a low risk of bias and 8 (80%) of these trials were randomized clinical trials. The remaining trials had “some concerns” in the risk of bias assessment. Table 1 summarizes the findings at the drug level.
Conclusion: Our cross-sectional analysis of FDA-approved hematological drugs from 2008 to 2022 provides valuable insights. Although many drugs received orphan and expedited approvals, trial design limitations were evident, with a majority being single-arm studies without comparators. Bias risk was observed, particularly in open-label non-randomized trials. These findings stress the significance of robust randomized controlled trials to support approvals and enhance evidence quality for hematological drugs. Addressing these issues will ultimately improve patient care and informed decision-making in the realm of hematology.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal